Corrosion is an incessant and costly problem. A recent study funded by the FHWA (Federal Highway Administration), NACE International and CC Technologies determined the total direct cost of corrosion is $279 billion per year, or 3.2 percent of the U.S. gross domestic product (GDP). Indirect costs (to the users/society) such as traffic delays, lost business, wasted energy are estimated to be almost five to ten times the direct cost, meaning the overall cost to society could be as high as 30 percent of the GDP.
The following is a corrosion tour of the city. Here you will see things you cross paths with everyday and, most likely, take for granted as natural, unavoidable, uncontrollable sights.
Pictured here is an obvious lack of maintenance of this corrosion prevention system - leading to ultimate project failure with apparent safety concerns.This is a very common sight — the complete failure of parking blocks due to unprotected reinforcing steel corroding.
This painted railing shows severe red-rust clumping. Not only is this unsightly, but it is extremely hazardous because the rusting is so extensive the railing is completely deteriorated in many locations.
This series of photos depicts two different corrosion situations: First, the concrete steps show staining and cracks in different areas. In the third photo, it is apparent the concrete was patched, which is an obvious repair of spalled concrete. Unfortunately, the patched area has stained and cracked again.
Second, while it’s difficult to see in the photos, the railing going up the steps is painted-over black steel with visible blistering, peeling, and rusting.
 Last on our city tour is a severely corroded beam from the famous Williamsburg Bridge — located in New York City and built in 1903. When this photo was taken, the bridge was still in use and traveled daily by over 100,000 vehicles; the lower level also accommodated train traffic. After more than 30 instances of this extensive corrosion were identified, the bridge was closed. The direct cost of the repair soared to over $750 million. The indirect costs are even more expansive: the loss of productivity due to the resultant traffic congestion, the loss of income by the businesses in the affected area, and the environmental impact from blasting are estimated to exceed the bridge repair’s direct costs by about ten times or close to $7.5 billion.
Last on our city tour is a severely corroded beam from the famous Williamsburg Bridge — located in New York City and built in 1903. When this photo was taken, the bridge was still in use and traveled daily by over 100,000 vehicles; the lower level also accommodated train traffic. After more than 30 instances of this extensive corrosion were identified, the bridge was closed. The direct cost of the repair soared to over $750 million. The indirect costs are even more expansive: the loss of productivity due to the resultant traffic congestion, the loss of income by the businesses in the affected area, and the environmental impact from blasting are estimated to exceed the bridge repair’s direct costs by about ten times or close to $7.5 billion.Why Unprotected Steel Corrodes?
So, why does unprotected steel corrode? Corrosion can simplistically be viewed as the tendency for any metal — in an elevated energy state such as a steel beam, plate, or bar — to revert back to its lower, more natural energy state of iron ore. This tendency is known as the Law of Entropy.
Here is a bimetallic couple model to help explain the corrosion process:
A bimetallic couple is the connection of two different metals (typical examples include brass or bronze valves connected to steel or cast iron pipes, stainless steel fasteners connected to steel or cast iron, etc.). Metals corrode via an electrochemical process; an electrochemical process is corrosion accompanied by a flow of electrons between cathodic and anodic metallic surfaces.
Four elements must be present at the same time for corrosion to occur: a cathode, anode, electrolyte solution, and a return current path (these terms are defined next). If you take away any one of these elements, corrosion will not take place.
• A cathode is an electrode* at which positive ions** are discharged, negative ions
are formed, or other reducing actions occur.
• An anode is an electrode at which negative ions are discharged, positive ions are
formed, or other oxidizing reactions occur.
• An electrolyte is a conducting medium in which the flow of current is accompanied
by movement of matter — most often an aqueous solution of acids, bases, or salts,
but may include many other media. An electrolyte is also a substance that is capable of forming a conducting liquid medium when dissolved or melted.
• The return current path is the metallic pathway connecting the anode to the
cathode. It is often the underlying substrate metal.
*An electrode is a conductor through which current enters or leaves an electrolytic cell.
**An ion is an electrified portion of matter.
So, in a bimetallic couple model with zinc and steel as the dissimilar metals, corrosion occurs at the anode (zinc). The cathode (steel) is protected from corrosion. This is how the term cathodic or sacrificial protection (zinc sacrifices itself to protect steel) is derived. The term anode corrosion may also be familiar to you.
The Solution to Corrosion
The problem of corrosion is clear. What is the solution?
As depicted earlier with the bimetallic couple, if all four required elements (anode, cathode, electrolyte, and return current path) are present, corrosion can occur. But, by isolating the metal from the electrolytes in the environment, the steel will be protected, and corrosion will not occur. This is known as barrier protection. Two important properties of barrier protection are adhesion to the base metal and abrasion resistance. Paint is perhaps the best known example of a barrier protection system, but has many limitations. Hot-dip galvanizing, applies an impervious zinc metal to steel, has extreme adhesion to the steel (3600 psi), and is highly abrasion resistant. However, it is not only a terrific barrier protection system, but also a cathodic protection system.
As has already been explained, zinc is anodic to steel; the galvanized coating will provide cathodic protection to exposed steel. When zinc and steel are connected in the presence of the electrolyte, the zinc is slowly consumed, while the steel is protected. The zinc’s sacrificial action offers protection where small areas of steel may be exposed due to cut edges, drill holes, scratches, or as the result of severe surface abrasion. Cathodic protection of the steel from corrosion continues until all the zinc is consumed. Hot-dip galvanizing is a factory-controlled process in which steel is protected against corrosion by a zinc coating applied by dipping the steel into a bath of molten zinc. This galvanizing process produces a durable, abrasion-resistant coating of zinc and zinc-iron alloy layers metallurgically bonded to the base steel and completely covering (inside and out) the piece of steel.
There are four basic phases to the galvanizing process:
1) Pre-inspection
When steel comes into the galvanizing plant, the material is first inspected to ensure proper vent and drainage holes have been provided (proper venting will be discussed in more detail shortly). Another part of the pre-inspection phase includes hanging the steel pieces on some sort of rack, chain, or wire system that will carry the material through the galvanizing process. The racks are lifted and moved through the process by an overhead crane system.
2) Cleaning
The steel must be thoroughly cleaned before it is galvanized, or the zinc will not bond to it. In this second phase of the galvanizing process, the steel goes through several cleaning steps.
The first step, caustic cleaning, consists of a hot alkali solution used to remove organic contaminants like dirt, water-based paint, grease and/or oil from the steel surface. All epoxies, vinyls, or asphalt must be removed by grit blasting, sandblasting, or other mechanical means before galvanizing. Removal of these materials is usually the responsibility of the fabricator, however, the galvanizer will do this if necessary. After caustic cleaning, the article goes through a clean water rinse. Next in the cleaning process, mill scale and rust are removed from the steel surface by pickling it in a dilute solution of heated sulfuric acid or an ambient temperature hydrochloric acid solution. After this step, the article goes through another clean water rinse.
The final step of cleaning, called fluxing, removes oxides and prevents further oxides from forming on the steel surface prior to galvanizing. Fluxing also promotes bonding of the zinc to the steel surface. The process of applying the flux to the steel depends upon whether the “wet” or “dry” galvanizing method is used. Dry galvanizing, the most common method, requires the steel to be dipped in an aqueous zinc ammonium chloride solution and then thoroughly dried before galvanizing. The wet galvanizing process uses a flux ‘blanket’ floating on top of the molten zinc. The final cleaning occurs as the material passes through the flux blanket before entering the galvanizing bath.
3) Galvanizing
The galvanizing phase of the process requires the steel material to be immersed in a molten zinc bath consisting of a minimum of 98% pure zinc. The bath temperature is maintained at approximately 850 F (approx. 455 C), and the metallurgical reaction and bonding of zinc to steel occurs when the material reaches the bath temperature.
4) Final Inspection
The fourth and final phase of the process occurs when the quality of the galvanized coating is determined through visual inspection and measurement of the zinc coating. As explained previously, if the steel surface is not properly and thoroughly cleaned, zinc will not react with and adhere to the steel and bare spots will be evident. During measurement the thickness of the zinc coating is magnetically calculated to ensure adherence to specifications.
Specifying for Design
Now that today’s severe corrosion problems and its various solutions have been discussed, it’s time to examine how to specify hot-dip galvanized steel in project designs. Protection against corrosion begins on the drawing board. No matter what corrosion prevention system is used, it must be factored into the design of the product. Once the decision has been made to use hot-dip galvanizing, the design engineer should ensure the steel pieces can be suitably fabricated for high-quality galvanizing.
Certain rules must be followed in order to design components for hot-dip galvanizing after fabrication. Adopting the design practices shown in the next couple of pages, along with those listed in ASTM A 385 Practice for Providing High Quality Zinc Coatings (Hot-Dip), will produce optimum quality galvanizing, reduce costs, assist with the timely processing of the product, and ensure the safety of the galvanizing personnel.
 The most important rule of designing for hot-dip galvanizing is the designer, fabricator, and galvanizer work together before the product is manufactured. This three-way communication will eliminate issues that could delay or prevent superior galvanizing quality. Most ferrous materials (those containing iron) are suitable for hot-dip galvanizing. Cast iron, malleable iron, cast steels, and hot- and cold-rolled steels all can be protected from corrosion by hot-dip galvanizing. Structural steel shapes, including those of high-strength, low-alloy materials are hot-dip galvanized to obtain long-lasting protection. Though most ferrous materials can be hot-dip galvanized, the chemical composition of the material affects the characteristics of the galvanized coating. During galvanizing, the ferrous material reacts with the zinc to form a series of zinc-iron alloy layers normally covered by a layer of pure zinc. For most hot-rolled steels, the zinc-iron alloy portion of the coating will represent 50 to 70 percent of the total coating thickness.
The most important rule of designing for hot-dip galvanizing is the designer, fabricator, and galvanizer work together before the product is manufactured. This three-way communication will eliminate issues that could delay or prevent superior galvanizing quality. Most ferrous materials (those containing iron) are suitable for hot-dip galvanizing. Cast iron, malleable iron, cast steels, and hot- and cold-rolled steels all can be protected from corrosion by hot-dip galvanizing. Structural steel shapes, including those of high-strength, low-alloy materials are hot-dip galvanized to obtain long-lasting protection. Though most ferrous materials can be hot-dip galvanized, the chemical composition of the material affects the characteristics of the galvanized coating. During galvanizing, the ferrous material reacts with the zinc to form a series of zinc-iron alloy layers normally covered by a layer of pure zinc. For most hot-rolled steels, the zinc-iron alloy portion of the coating will represent 50 to 70 percent of the total coating thickness. 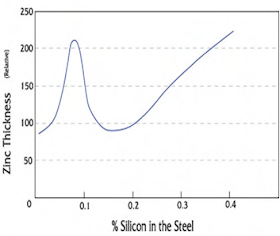 Steel compositions vary depending on strength and service requirements. Major elements in the steel, such as silicon and phosphorus, affect the galvanizing process, as well as the structure and appearance of the coating. For example, certain elements present in the steel may result in a coating composed almost entirely, or completely of zinc-iron alloys. Per ASTM A385, Section 3.2, certain elements found in steels are known to have an influence on the coating structure. Carbon in excess of about 0.25%, phosphorus in excess of 0.04%, or manganese in excess of about 1.3% will cause the production of coatings different from the normal coating. Silicon concentrations between 0.05 - 0.15%, or above 0.25% have a profound effect on the nature of the coating produced (see chart ).
Steel compositions vary depending on strength and service requirements. Major elements in the steel, such as silicon and phosphorus, affect the galvanizing process, as well as the structure and appearance of the coating. For example, certain elements present in the steel may result in a coating composed almost entirely, or completely of zinc-iron alloys. Per ASTM A385, Section 3.2, certain elements found in steels are known to have an influence on the coating structure. Carbon in excess of about 0.25%, phosphorus in excess of 0.04%, or manganese in excess of about 1.3% will cause the production of coatings different from the normal coating. Silicon concentrations between 0.05 - 0.15%, or above 0.25% have a profound effect on the nature of the coating produced (see chart ).Duplex Systems
For years, protecting steel from corrosion typically involved either the use of hot-dip galvanizing or some type of paint system. However, more and more corrosion specialists are utilizing both methods of corrosion protection in what is commonly referred to as a duplex system. A duplex system is simply painting or powder-coating steel that has been hot-dip galvanized after fabrication. When paint and galvanized steel are used together, the corrosion protection is superior to either protection system used alone. Painting galvanized steel requires careful preparation and a good understanding of both painting and galvanizing. The ASTM specification for preparing galvanized steel surfaces to be painted is D 6386.
The galvanized coating protects the base steel, supplying cathodic and barrier protection. Paint, in turn, grants barrier protection to the galvanized coating. The paint slows down the rate at which the zinc is consumed, greatly extending the life of the galvanized steel. In return, once the paint has been weathered or damaged, the zinc is available to provide cathodic and barrier protection so rust will not develop on the substrate steel.
Rebar
Corrosion and repair of corrosion damage are multi-billion dollar problems. Observations on numerous structures show that corrosion of reinforcing steel is either a prime - or at least an important – factor contributing to the staining, cracking, and eventual spalling of concrete structures. These effects of corrosion often require costly repairs and continued maintenance during the life of the structure.
Epoxy-coated rebar can experience the same type of corrosion at places in the concrete where the epoxy coating is defective, or at exposed cut ends of the rebar.
When hot-dip galvanized rebar is used, the zinc corrodes in the same process as bare steel, but at a much slower rate. Unlike the very dense corrosion by-products of bare steel, zinc corrosion by-products are significantly less dense than iron oxides. The zinc corrosion byproducts are powdery, non-adherent, and capable of migration from the surface of the galvanized coating into the matrix of the concrete, highly reducing the likelihood of zinc corrosion-induced spalling.
Galvanized rebar has superior bond strength essential for reliable performance of reinforced concrete structures. These studies performed by the University of California – Berkeley show galvanized rebar to have greater bond strength, measured by stress in pounds-persquare- inch, than deformed black steel. The majority of the pullout strength comes from the ribs on the bar, but these studies show extra pullout strength can be realized with galvanized rebar.
This unique structure - known as “The Egg” is a wonder of modern construction. The “shell” is shaped by a heavily reinforced concrete girdle, which helps keep “the Egg’s” shape and directs the weight of the structure to the supporting pedestal and stem. Adding even more durability to this structure are miles of galvanized rebar weaving in and out of the shell and stem. “The Egg” was constructed in 1966 and took 12 years to build. Today it remains a beautiful piece of rust-free architecture.
Any exterior environment is prime for the use of hot-dip galvanized steel. Common applications include theme park rides, stadiums, utility towers, bridge girders and decks, ornamental fences, docks, boat trailers, anchoring rods, bolts and nuts, handrail, grating walkways, and stairs.In addition to the long-term corrosion protection it offers, hot-dip galvanizing is chosen because it provides maintenance-free performance for decades.







